Introduction:
Ibrutinib works by inhibiting downstream signaling after the interaction between the mutated MYD88 (Leu265Pro) protein and BTK. In 2015, Ibrutinib, an inhibitor of Bruton's tyrosine kinase (BTK), was approved by the U.S Food and Drug Administration (FDA) for patients with symptomatic Waldenstrom Macroglobulinemia (WM).
Methods:
We performed a comprehensive literature search following PRISMA guidelines. Beginning with articles published after April 2015, we used databases like PubMed, Embase, Clinicaltrials.gov, Cochrane Library, and Web of Science. A total of 580 articles were identified initially, and after a detailed screening, we finalized 4 studies involving 248 WM patients.
Results:
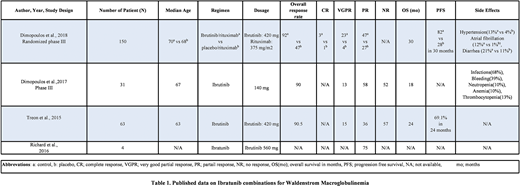
The total number of patients who received Ibratunib based regimens were 248. The dose ranged from 140-560 mg. The overall response rate ranged from 90-92%, very good partial response ranged from 13-23%, and the partial response ranged from 36-75%.
1) Ibtrutunib +Rituximab:
In a study by Dimopoulos et al., (N=150), the overall response rate (ORR) was 92% vs 47% in control vs placebo respectively. The progression-free survival (PFS) of 30 months was 82% vs 28% respectively.
2) Ibrutinib alone:
In a study by Dimopoulos et al., with (N=31), ORR was 90%, a very good progression rate (VGPR) was 13 %, and the partial response (PR) was 58%. In a study by Treon et al., (N=63) ORR was observed in Treon et al. at 90.50%, VGPR was 15%, PR was 36%, no response (NR) was 57%, with overall survival (OS) was 24 months. In a study by Richard et al., (N=4), PR was noted at 75%.
Conclusion:
Ibtrutunib used for the treatment of WM showed an overall response rate of 92%. Infections, cytopenia, bleeding, hypertension and atrial fibrillation were major side effects reported. However, due to the recent FDA approval of the drug to be used for the patients of WM there is very limited studies on Ibrutinib. We recommend future randomized prospective trials to understand the efficacy and safety profile of Ibrutinib for WM treatment.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.:Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal